Identification of medical devices – UDI
(Unique Device identification)
 Penteq is GS1 solution Provider
Penteq is GS1 solution Provider
Since 24 September 2014, medical devices must be clearly identified by the UDI directive when they are delivered to the US market.
GS1 is an accredited organization for the identification of products and offers practical solutions with the GS1 standards to meet the requirements.
In order to meet the requirements of medical technology products, a permanent marking is required.
The following criteria must be ensured:
- Clear and complete traceability
- Products must be permanently labelled
- No increased corrosion of stainless steels
- Must resist cleaning and sterilization processes
- Each code must be used only once
Through the UDI, a complete traceability of medical devices is ensured by a clear and internationally valid identification.
It also ensures the identification of medical devices throughout the life cycle.
Required callbacks, as well as messages in the context of unwanted events, can be performed much more efficiently.

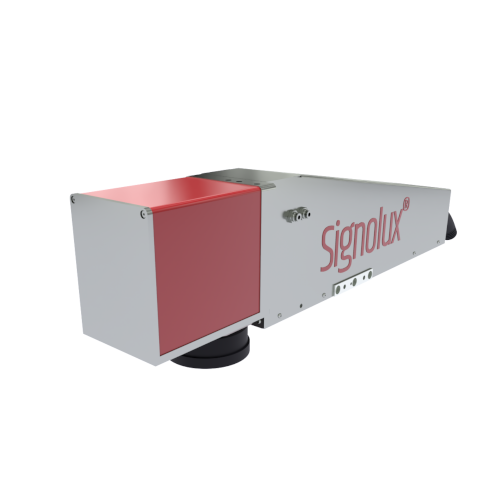
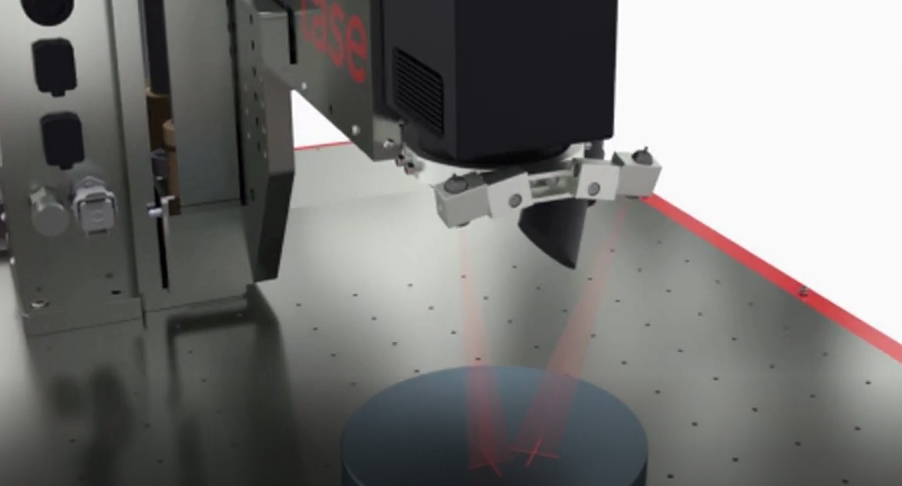
PenMedLase 50
The PML 50 Desktop laser System is a compact and user-optimised system of laser Class 1 and specially designed for applications in medical technology.
The robust and powder-coated steel construction of the protective cabin in combination with stainless steel is designed as the complete system for long-term use.
PENTEQ supports you from initial contact to the provision of standards-compliant codes.
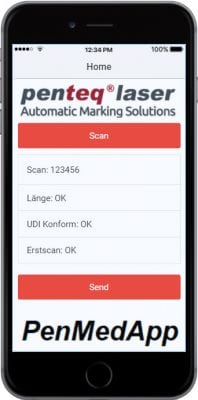
PenSoft Addon Medical App (only in combination with PENMEDAPP): To check whether the code is actually applied to the product, the user interface is locked after the labeling process until the operator "proves" that the code was actually applied to the product in a readable way. . The proof can be made in two ways:
The operator starts the PENMEDAPP developed specifically for this case and scans the applied code with the smartphone. The smartphone or app is connected to the user interface and switches it off again when the check is successful and the next product can be processed.
PENMEDAPP:
The app developed specifically for the medical sector takes care of the control of the applied codes on the product. The app is connected to the machine or the operating software and data can be exchanged.
In the app, a code reader is implemented, which reads the applied codes and propagates the content to the machine. The machine checks whether the content is the same as that which is to be on the product and releases the surface for further processing. Query status information such as operating hours, total number of pieces, number of pieces per shift, good parts, bad parts are possible.
Our app for your smartphone is available for download on Android and iOS (in compliance with the minimum requirement).
Support through remote maintenance can be offered if there is a network connection on the customer side.