Medical technology - UDI
Laser marking for durable and traceable labelling
UDI compliant - laser labelling on medical devices
Durable and clear labelling for the highest demands
Ultra Black Marking is a special type of laser marking in which ultra-short laser pulses from the Signolux® laser in the picosecond or femtosecond range are used to create an extremely dark, high-contrast and corrosion-resistant marking. The colour is created by selectively influencing the surface structure at nanometre level - without any conventional engraving or roughening.
This creates permanent markings with UDI codes and serial numbers.
Ultra Black Marking with UKP laser Signolux® in medical technology
The advantages of the laser lie in the wide range of applications and the outstanding labelling quality.
Permanent, high-contrast marking without damaging the material
Maximum precision and traceability are essential in medical technology.
The ultra-short pulse laser technology (USP) of the Signolux® laser enables an innovative process for permanent black marking - so-called Ultra Black Marking. This method ensures stable, legible markings on stainless steel, titanium and other medical materials - without any material removal or structural changes.

Penteq is a GS1 Solution Provider
As an official GS1 Solution Provider, Penteq supports companies in implementing global standards for labelling and traceability.
With our many years of experience in laser technology, we offer customised solutions for industrial direct marking - precise, durable and standard-compliant.
Our systems enable the integration of GS1-compliant codes such as DataMatrix or barcodes directly into your production processes.
What does UDI-compliant labelling mean?
Unique Device Identification (UDI) is a globally mandatory system for the unique identification of medical devices. The requirements are defined by the following regulations, among others EU-MDR 2017/745 (Europe) FDA UDI Rule (USA) GS1, HIBCC or ICCBBA as standard data formats.
A UDI consists of two parts: the device identifier (DI) and the production identifier (PI). The labelling must be machine-readable (e.g. DataMatrix code) and in plain text - permanently and clearly legible over the entire product life cycle. The Signolux® laser is therefore ideal for marking the UDI in accordance with the new EU Medical Device Regulation.
Example of the validation of a laser system:
As a medical company, would you like to ensure that your laser correctly applies data matrix codes to surgical instruments?
The following test steps should be carried out:
Is the code always legible?
Is the code permanent?
Is there a verification system in place?
Is every step documented?
Is the software protected against changes?
➡ We actively support your validation processes and guide you towards a standard-compliant process.
Validation in accordance with GMP and GAMP is a structured process that ensures that a system (e.g. a laser system for labelling) works reliably and reproducibly - especially in regulated industries such as pharmaceuticals, medical technology or biotechnology.
Why Signolux® laser labelling?
Laser marking with the Signolux® laser is the preferred method for medical products - especially for stainless steel and glass.
Thanks to the special physical properties of the laser, stainless steel can be marked with high contrast and durability without negatively changing its properties. Glass is labelled without introducing microcracks into the material structure, thus ensuring durability and the highest quality.
Tamper-proof & durable
The laser markings are abrasion-resistant, corrosion-resistant and suitable for sterilisation.
Contactless & gentle on materials
Ideal for sensitive components - without chemical additives or inks.
Highest precision & quality
Detailed engraving of logos, serial numbers, data matrix codes Nanostructured surfaces that scatter light to create a deep black effect.
Minimal heat exposure, which is particularly advantageous for heat-sensitive materials.
No oxide layers that could change or corrode over time
The advantages of laser labelling in medical technology
Areas of application
- Surgical instruments
- Implants and prostheses
- Endoscopic devices
- Diagnostic and laboratory technology
- Plastic housings for medical electronics
- Glass containers
Forgery-proof
- Tampering leaves visible traces
- Clearly identifiable through Data Matrix codes
Precise
- Outstanding labelling quality
- High contrast and high read reliability
Resistant
- Highly resistant to heat, abrasion and mechanical stresses
- Withstands sterilisation processes
- Suitable for establishing a traceability system

Suitable products

Integration station
Laser system for easy integration into conveyors

VisionSystem Verifier
Verification of e.g: Data Matrix codes, barcodes, QR codes, etc.

Smoke extraction systems
Reliably extract and filter laser smoke, laser particles and fumes
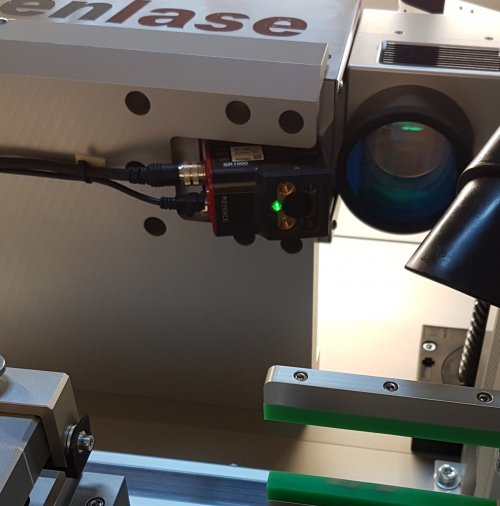
VisionSystem Check and Confirm
Checking data matrix codes, barcodes, QR codes by reading against them

Signal light
Reliable signalling for maximum visibility and safety
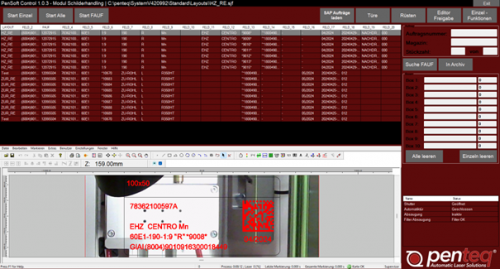
PenSoft Connect IP
Software für die Typenschildproduktion

Schwenkarm
Komfort und Funktionalität in perfekter Harmonie

Hand scanner
Powerful, flexible and reliable

Foot switch
Foot switch for Penteq laser machines







